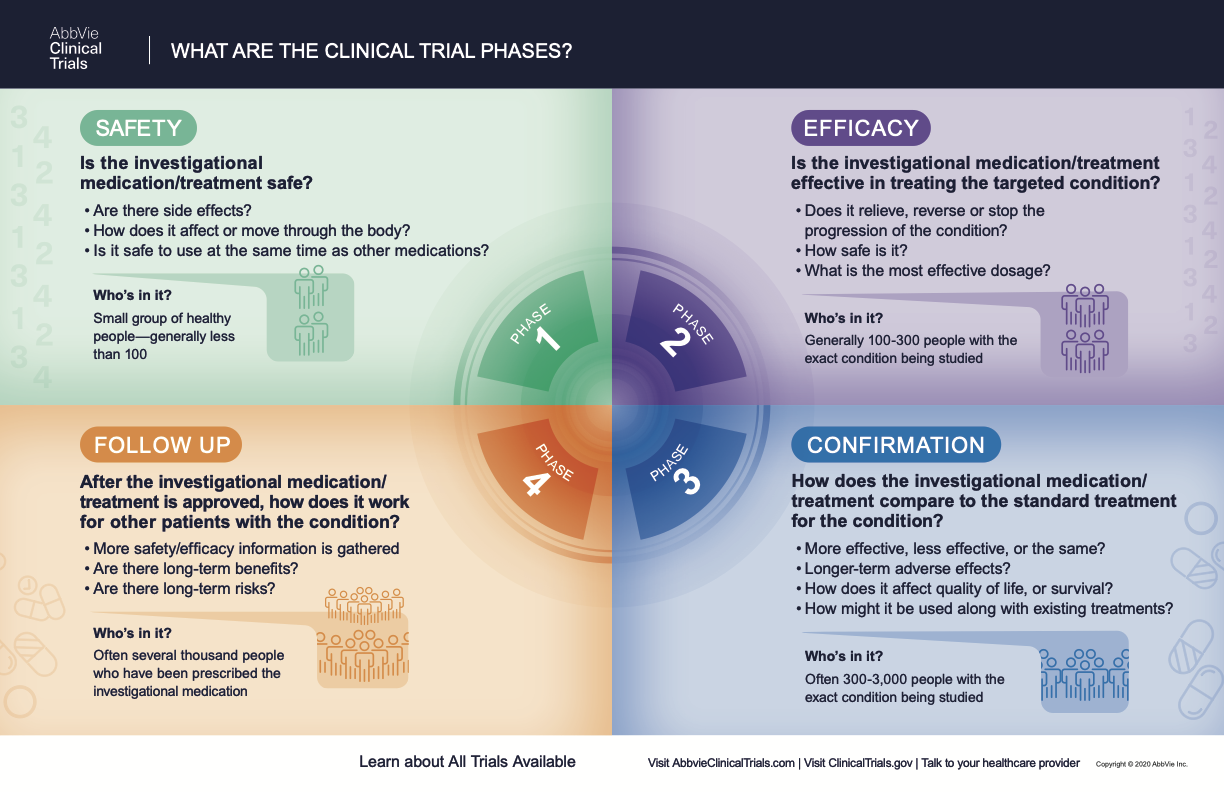
CLINICAL RESEARCH PHASES
Clinical trials follow a particular timeline, from early, small-scale, phase 1 studies to late-stage, large-scale, phase 3 studies.1 While there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. Here we describe the key goals and provide information about the various clinical research phases.
Preclinical Studies
Before a clinical trial begins, researchers perform extensive preclinical studies in the lab to make sure that their methods (e.g., drug, procedure, preventative measure, or diagnostic) are not harmful to people. The level of harm is measured in terms of toxicity.
The research in preclinical trials is not performed on people. Instead, potential drugs and therapies, and the methods to administer them, are first tested in cells or animals, or both, long before they make it to human trials.
Usually, preclinical studies are not very large. However, these studies provide detailed information on dosing levels and are required before clinical trials can begin.
After preclinical testing, researchers review their findings and decide whether the method should be tested in people. If the treatment appears safe in cells or animals, it proceeds to phase 1, where the potential medical treatment is first tested in humans.
Phase 1 Clinical Trial Definition
Once the preclinical studies have shown that a clinical method may work and appears safe, it is tested on a very small group of healthy volunteers for a few hours or days, up to a few months or even to a year or two.2 If the clinical trials are investigating cancer or rare diseases, the phase 1 trials may enroll these patients rather than healthy volunteers.
In the case of potential medical treatments or drugs, a phase 1 study determines safety for humans in a few different ways including side effects, how the body absorbs, distributes, and eliminates the drug, and whether it is safe to use in combination with other medications.
At the end of phase 1, if the study treatment and method of administration appear safe in healthy people, the next phase of development is phase 2 where it is tested on a slightly larger number of people with the targeted disease. According to the FDA, an estimated 70% of drugs move from phase 1 to phase 2.1
What Happens in Phase 2?
Phase 2 clinical trials build on the results of phase 1 by testing the method on participants with the health condition targeted in the study. For example, phase 2 clinical trials will begin to focus on a specific type of cancer, such as acute myeloid leukemia or glioblastoma, or a specific neurodegenerative disease like Alzheimer’s disease. What happens in phase 2 trial is similar to what happens in phase 1, except that the duration of the study and the number of participants is greater and some of the information measured is related to the how the new method or medication works against the target health condition. Researchers are looking for the efficacy and safety of the clinical method over a period of time longer than phase 1.1,2 The number of participants is increased in phase 2 to around 100 to 300 tested.
When a potential treatment is being studied, participants are closely watched for how effective the study treatment is on the targeted health condition to determine how safe it is for the patient.
In randomized trials, researchers want to determine the best option among several choices, and they randomly assign each patient to a particular study treatment and compare the results between the different study treatments.
Trials can also be blinded, meaning that the participants do not know what study treatment they are receiving to ensure effectiveness and impartiality of the study treatment. Some trials are placebo-controlled, meaning that some participants will get a placebo or “sugar-pill” and not the medication or study treatment under investigation. Other trials are active-controlled, meaning that some participants will get a study treatment that is already available for the targeted health condition.
It is common for clinical trials in phase 2 to be randomized, blinded, and placebo- or active-controlled to ensure finding the best study treatment available.
Phase 2 study treatments are typically performed in an outpatient setting, but there are clinical trials that may require participants to stay overnight in a hospital or medical center for additional monitoring.
If phase 2 has tested efficacy, safety, and optimal dosage with positive results, the drug can move on to phase 3 testing in more participants for even longer. Approximately 33% of phase 2 studies move to phase 3.1
However, most clinical trials fail in phase 2 because the study treatment cannot be shown to be effective in people with the targeted condition.
What is a Phase 3 Clinical Trial?
Phase 3 clinical trials are designed to test whether the investigative treatment is better than the standard clinical method for the targeted health condition. Usually two or more phase 3 trials are conducted. The most reliable study design is a randomized, blinded and placebo- or active-controlled trial to verify that the results are beneficial.
Phase 3 studies are tested over a longer period than phase 1 or phase 2 studies and include many more participants with the targeted disease, often between 300 to 3,000.1 By testing more participants, researchers can more confidently see if the investigational treatment has any adverse effects.
While a study treatment is being examined, participants are closely monitored for side effects, longer-term effects, and possibly other things such as quality of life or survival. By testing multiple study treatments, researchers can see, for example, how new and existing treatments can be best used to treat the health condition in question.
If phase 3 is completed successfully, as are approximately 25%-30% of such studies,1 the new method or study treatment can be submitted to the regulatory agency for approval and eventual use by the general population. Once approved, the new medication can then be marketed in the United States.
What Happens in Phase 4?
Phase 4 clinical trials collect results after a medication has been introduced into the general population to see how well it works on “real life patients” in order to determine the long-term benefits and risks.
Most often, phase 4 studies are observational studies that collect data from real-life patients who are taking a medication as prescribed by their doctors. Phase 4 clinical studies are usually performed by the pharmaceutical or biotechnology companies that manufacture the study treatment.
Sometimes, regulatory agencies like the FDA approve a treatment for marketing only if the effects of the treatment are further monitored in phase 4 trials. This may happen if previously untested groups of patients experience adverse reactions to the treatment.
These findings are compared with all the results from the previous trial phases to make sure that the recently approved treatment is safe and effective.
What to Look for When Searching for Clinical Studies
From the name of the trial to the last words on the page, looking at a clinical study recruitment website or form can be intimidating. Likewise, understanding the clinical trial phases, common acronyms, and procedures involved in a study may be confusing. A clinical trial posting should provide key information such as who is conducting the trial and why, the location of the trial, basic eligibility requirements and contact information so that participants can determine if a clinical research study is a good option.
Clinical research trials are usually categorized by disease name or area and typically provide a short description of the trial. The information about clinical trial phases, the purpose of the trial, and the type of participants the investigators are hoping to recruit will generally be presented as:
“This is a phase 1 (2, 3, or 4) study evaluating safety, efficacy, pharmacokinetics, or dosing in healthy or diagnosed subjects.”
Clinical trial postings are usually straightforward about the type of participants being sought for the study, either healthy or with a specific condition, and their desired age and gender, if relevant. They will also include the study start and end date so that if it doesn't work with your schedule, you can find a trial better suited to your lifestyle.
If you need more information about the clinical trial, most postings are accompanied by a reference section for additional trial requirements and contact information to quickly make a decision about whether a trial is a potential fit for you.
Scientific terminology is often the most daunting part of clinical trial phases, as well as their inclusion and exclusion criteria, which are highly detailed requirements for participation.